Incontinence after prostatectomy? Are you interested in speeding recovery and reducing leaks?
At-home Research Study for Urinary Incontinence in Men
What if you Could Speed Recovery?
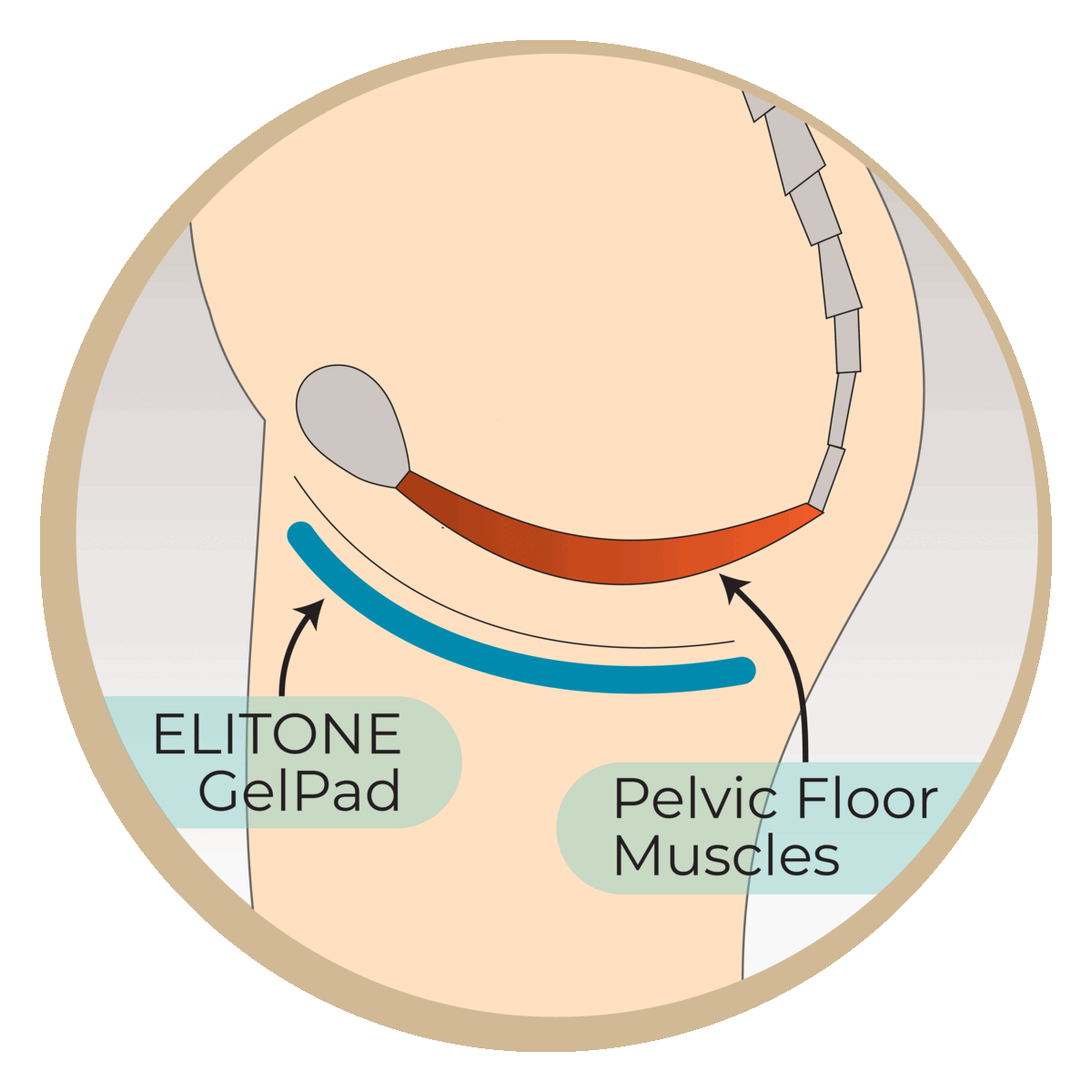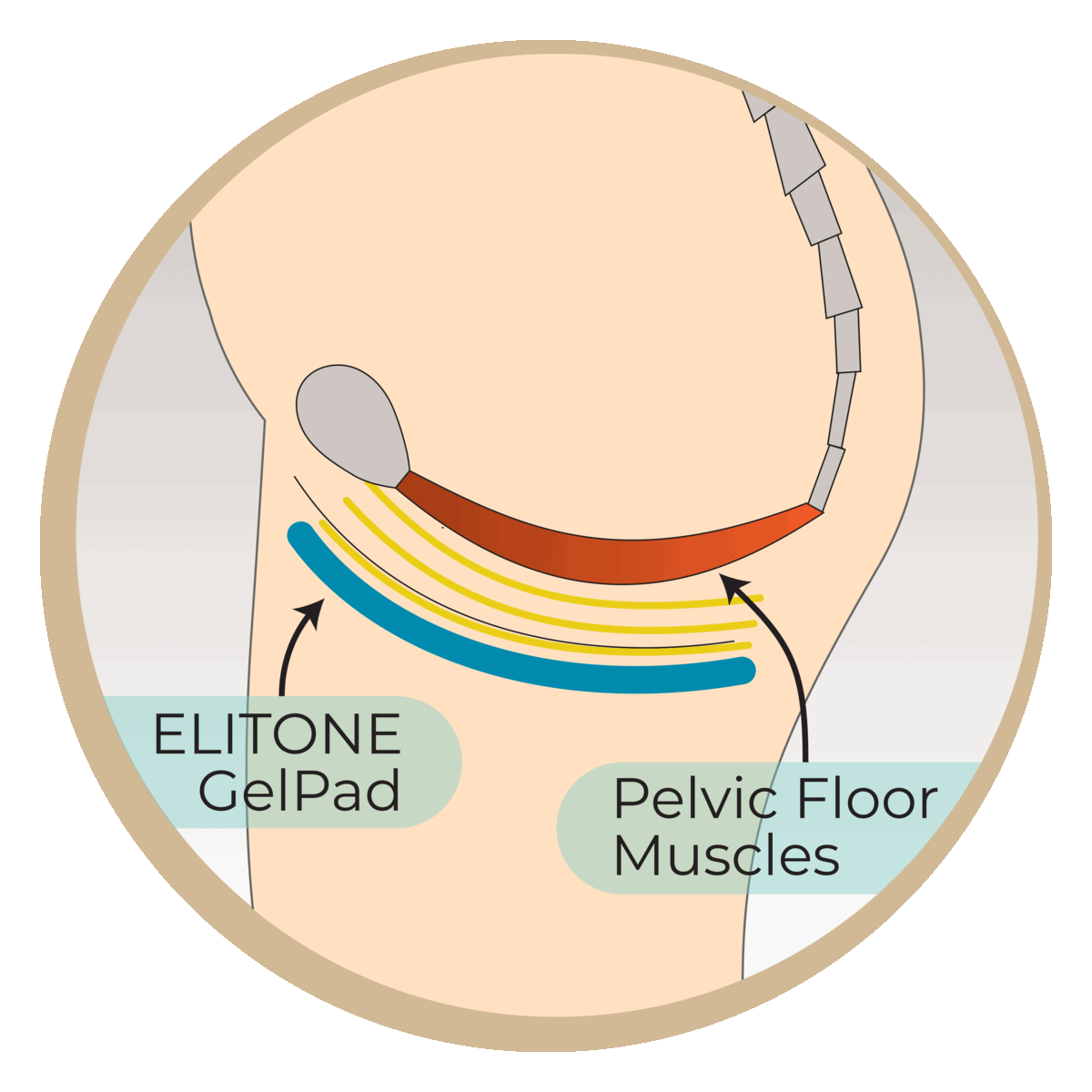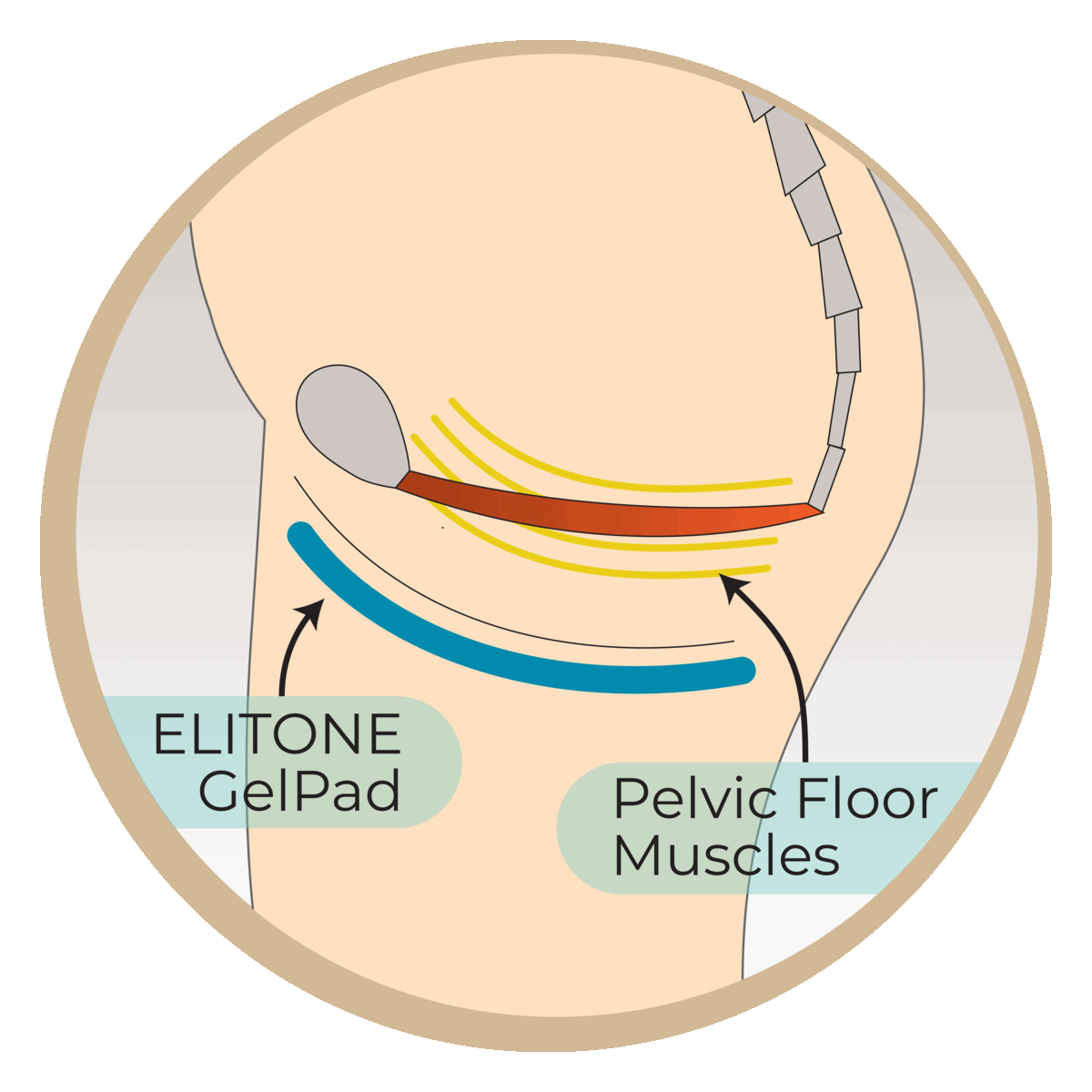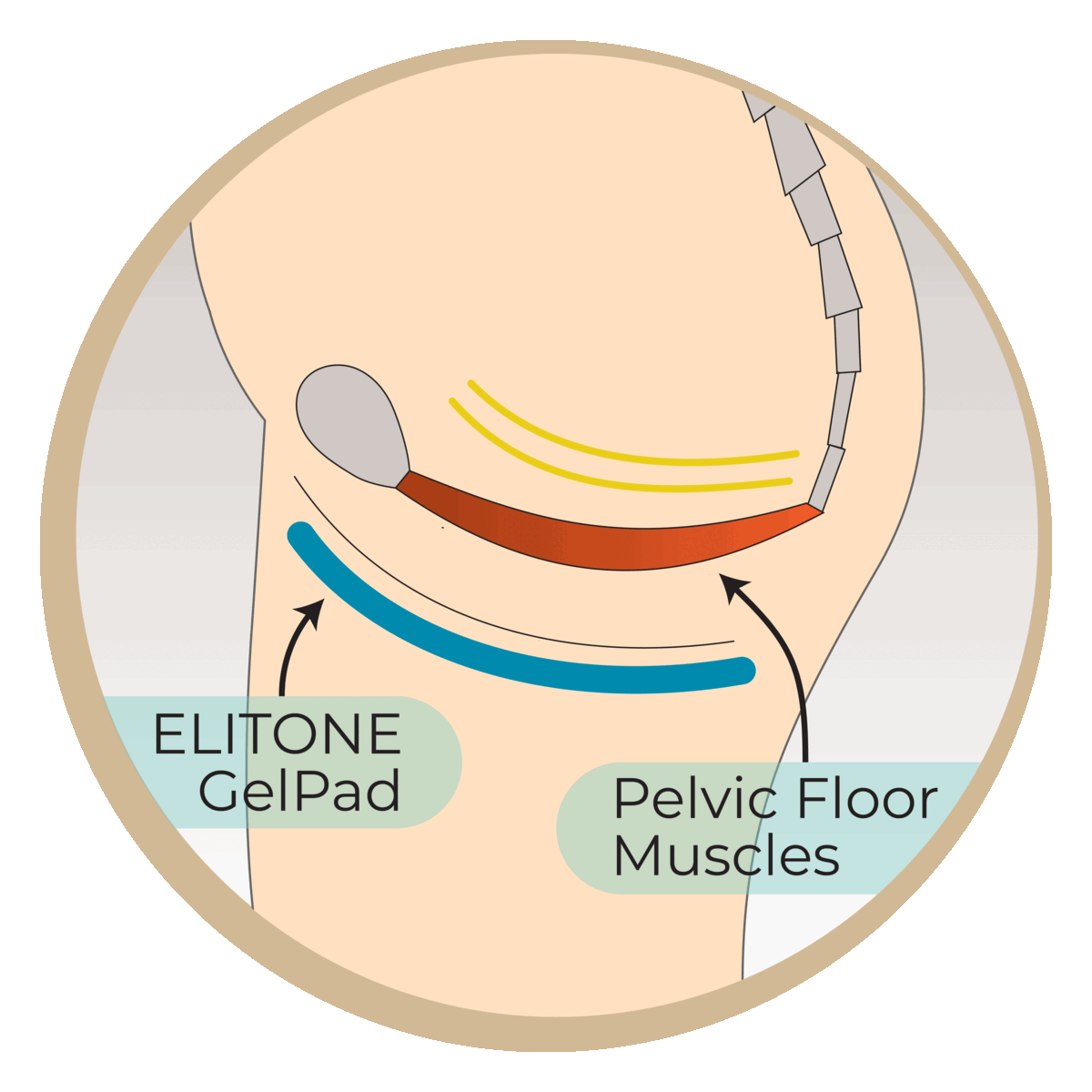
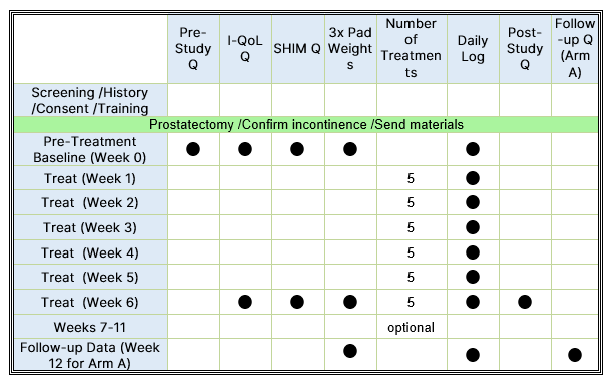
Studies have shown that almost half of men are still wearing pads at 6 months post-surgery. This research study aims to evaluate the Elidah wearable treatment device’s effectiveness in reducing incontinence and pad-use in men post-prostatectomy, and speeding recovery of sexual function, and confidence. The device uses neuromuscular stimulation to externally tone pelvic floor muscles.
Incontinence after prostatectomy affects sexual function. Did you know that studies show only 14.6% of men could have erections firm enough for intercourse at 12 months post-prostatectomy?1 Unfortunately, recovery of normal urinary control can take 1-2 years in some patients.2